Continuous monitoring of in-line oxidation reduction potential assisted a major copper producer in developing a sustainable, efficient approach to slimes processing that required less costly reagents, increased yield and improved efficiency.
By Daniel Kim and Shijie Wang, Rio Tinto Kennecott Utah Copper; and Doug Brees, Mettler-Toledo Ingold Inc.
Sustainable development as a concept and as a goal has spread rapidly and is now an integral facet of the operations of most major mining enterprises. With an emphasis on values and goals, mining companies focus on maximizing resources and positioning themselves to take advantage of technological development, while still considering sustainable development in regard to profitability, safety and operating efficiency.
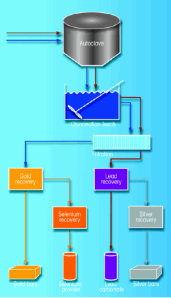 |
| Figure 1: Material flow diagram of slimes processing in the precious metal plant. |
At Rio Tinto Group’s Kennecott Utah Copper (KUC), a focus on economic prosperity, social well-being, environmental stewardship, and strong governance systems has been practiced within its business model since 2005. Understanding these practices and being able to identify projects that will contribute to sustainable development is key to the company’s long term success and ability to continue operations. KUC’s intention is to get to a point where everything the company does is driven by sustainable development.
A refinery modernization program undertaken in the mid-1990s at KUC led to an increase in tank house production capacity from 200,000 metric tons per year (mt/y) to 310,000 mt/y (850 mt per day average). Cathode quality and appearance improved even though the anode impurity levels increased, unit operating costs were reduced through increased productivity and safety, and environmental protection also improved. More recently, through implementing multiple-step Six Sigma processes, the refinery has further enhanced its productivity, produces higher quality copper, increased its profitability, and met stringent customer demands. This article describes KUC’s slimes process improvements, in particular wet chlorination for gold leaching.
Kennecott Copper Anode Slimes Process
The KUC refinery is located at Magna, approximately 16 miles west of Salt Lake City, Utah, USA. In the refinery’s tank house, commercial anodes from the KUC smelter are electrorefined. When a copper anode dissolves during electrorefining in the refinery tank house, slimes (insoluble material) are continuously released from the anodes and deposit at the bottom of the cells. These insoluble metals and compounds comprise various amounts of copper, gold, silver, selenium, tellurium, lead, bismuth and barite, together with traces from the platinum group metals.
The slimes are then pumped to the precious metals (PM) plant as a slurry in a mixture of drain electrolyte, cell and floor wash water, and anode / cathode wash water. In the PM plant, the anode slimes are initially thickened in three settling tanks, each of 40,000 gallon (151,000 liter) capacity. The liquid phase is decanted and filtered through two plate and frame filters. When the filtered solution is clear enough it is returned to the tank house as electrolyte make-up solution.
Next, the electrolytic refinery slimes are leached with sulphuric acid and oxygen in two autoclaves to achieve rapid and complete oxidation of the copper present in the slimes. The decopperized slimes then go through a wet chlorination process. In the following step, the leach solution is fed to a solvent extraction process that includes two-stage countercurrent extraction with dibutyl carbitol (diethylene glycol dibutyl ether) and multi-stage countercurrent scrubbing with 1–3 molar hydrochloric acid. Gold is reduced directly from the organic phase by contact with an aqueous solution of the reducing agent, and crude selenium is recovered from the gold-free raffinate by reduction to its elemental form. Finally, wet chlorination residue is re-pulped in a dilute solution of sodium carbonate and leached with a solution of aqueous ammonia for lead and silver recovery (See Figure 1).
Wet Chlorination
Slimes at Kennecott have a relatively high gold to silver ratio of 1:10, hence hydrometallurgical processing through chlorination was chosen to allow for production of gold.
Ag2Se + 2HCl + 3H2O2 H2SeO3 + 2AgCl + 3H2O (2)
2Au + 8HCl + 3H2O2 2HAuCl4 + 6H2O (3)
2HCl + H2O2 Cl2 + 2H2O (4)
H2O2 1/2O2 + H2O (5)
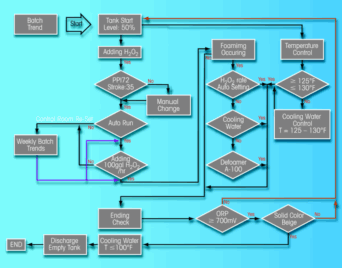 |
| Figure 2: Control chart of wet chlorination process. |
Wet chlorination of decopperized slimes is achieved through the metered addition of hydrogen peroxide to agitated slurry of slimes suspended in an aqueous solution of hydrochloric acid. The process of wet chlorination oxidizes and solubilizes the gold and selenium in the slimes. Hydrogen peroxide is used as the oxidant and hydrochloric acid provides the chloride for gold complex solubility. Side reactions that occur during this leach include solubilization of some slime impurities and conversion of lead sulfate to lead chloride. Process chemistry occurs via the following reactions:
Although chlorine is a considerably cheaper oxidant than hydrogen peroxide, it was not chosen to be used in the wet chlorination for safety reasons as chlorine is an extremely dangerous material to store, transfer and deliver to the chlorination tank.
Continuous Improvement
The chlorination step is the heart of the precious metals recovery process. It is of such importance in the PM plant that it could affect the enterprise performance of KUC, not only in the number of gold bars produced, but it could also leave selenium in the leach residue, creating quality issues. One of the main problems that can occur during chlorination is rapid foaming of the tank content. When foam rises to a certain level in the leach tank, addition of reagents must stop. This delays the chlorination cycle resulting in a bottleneck in PM operations.
To optimize this process, a Six Sigma process was carried out with efforts that focused on temperature control and dosage or reagents such as hydrogen peroxide, based on the oxidation reduction potential (ORP). This process solved the foaming issue and the reaction time was reduced.
With the Six Sigma continuous improvement methodology, a wet chlorination process control chart was drawn and the process was implemented (See Figure 2). This process has increased the gold bar/byproducts ratio significantly, and resulted in additional revenue and an increase in the chlorination process capacity.
Fundamentals of ORP
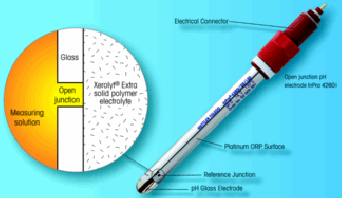 |
| Figure 3: Illustration of a pH/ORP electrode. |
As mentioned above, other than temperature, a major control of wet chlorination is through the monitoring of the ORP (or redox) potential. An ORP reading provides an indication of the ability of a solution to support oxidation or reduction. As the name implies, this is a potentiometric (voltage/millivolt) method. ORP electrodes contain both a reference electrode and a measuring electrode. The sensor used is in fact a combined pH/ORP sensor (See Figure 3).
The reference electrode is identical to that used for pH, and as its name implies, serves as a stable reference voltage to compare the measurement electrode reading. The measurement electrode for ORP is platinum metal and for pH it is a glass electrode. The inert platinum electrode does not chemically react with the solution, but senses the electron “pull” of oxidizing solutions or the electron “push” of reducing solutions. The presence of oxidizing chemicals causes the millivolt reading to be more positive (+), while presence of reducing solutions cause a negative (–) reading. ORP measurement therefore provides a direct indication of the oxidative strength of a solution.
ORP Measurement In-Line
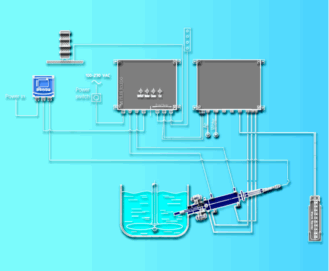 |
| Figure 4: Illustration of the ORP measurement system. |
ORP measurement is important during wet chlorination as it indicates when complete dissolution of the gold content of the slimes has been reached. Therefore, it prevents on the one hand, gold being carried in the raffinate, and on the other, excessive and costly chlorination. The oxidation potential is monitored by in-line ORP measurement. For reliability and accuracy purposes Mettler Toledo ORP measurement systems were installed.
The high suspended particles content in wet chlorination can quickly interfere with the performance of ORP sensors due to particles forming a coating on the sensor’s surface and process chemicals entering and poisoning the sensor’s reference electrode. To counteract this, the chosen sensor features a non-clogging open reference junction and a solid polymer electrolyte that resist poisoning. To ensure the sensor is always operating optimally, it is used in conjunction with an automated cleaning system. Using a retractable sensor housing, the system automatically extracts the ORP sensor from the chlorination process to perform cleaning when required. The diagram in Figure 4 shows the integration of the EasyClean cleaning system into the measuring point. A real-time recording of the ORP measurements vs. H2O2 dosing is shown in Figure 5.
Higher integrity of the measurement is obtained through the use of Mettler Toledo’s Intelligent Sensor Management (ISM) concept. This technology comprises pH/ORP sensors with embedded microprocessors. One advantage of ISM is that the sensor output signal is fully digital and therefore cannot be corrupted by wet electrical connections, electromagnetic interference, or long cable runs. Another benefit is that ISM sensors feature predictive diagnostics. Thanks to these, measurement availability can be ensured at all times, as possible measurement problems are detected before they become significant. The sensor will, for instance, indicate how many days of reliable operating life are left so that action can be taken before the sensor fails.
Several ISM pH sensors are installed in the side processes of the wet chlorination, all of which require very little maintenance time compared to conventional analog sensors.
Competitive Advantage from ORP Measurement
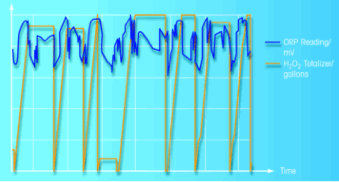 |
| Figure 5: ORP measurement in-line. |
Technology developments have reduced the production costs for all commodity metals over time at KUC, either through significant step changes, such as smelting and refining modernization, or by continuous improvement implementations, such as the anode slimes, wet chlorination operation described above. Through implementing multiple-step Six Sigma tools in processing of the copper anode slimes and the technology development of in-line, intelligent ORP measurement in the wet chlorination operation, the refinery has enhanced its productivity, produces higher quality copper, gold and other metals, and increased its profitability. For KUC, technology developments have overcome challenges, and sustainable competitive advantages have been achieved.









